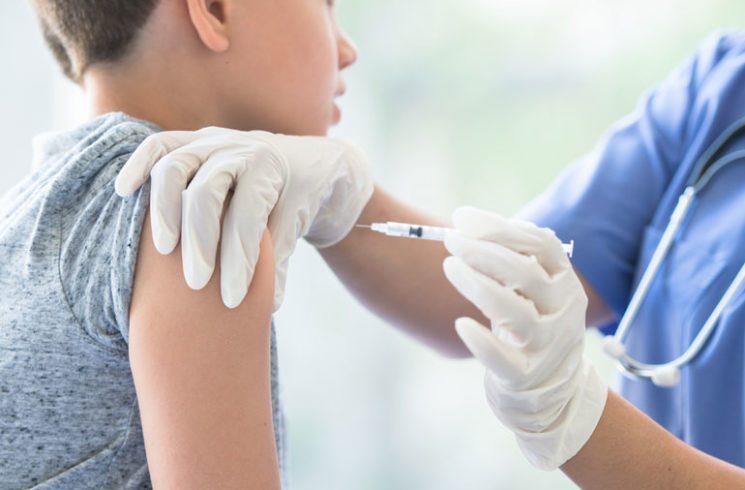
The FDA has approved Pfizer and BioNTech’s request for the emergency use of the Covid-19 vaccine on those ages 12 to 15.
The United States Food and Drug Administration (FDA) has approved Pfizer and BioNTech’s request for the emergency use of the Covid-19 vaccine on adolescents.
Pfizer and BioNTech submitted in April a request to the FDA to expand the emergency use authorisation of its Covid-19 vaccine, enabling kids aged 12 to 15 to receive the injection.
The pharmaceutical company has been conducting countless trials to ensure the safety of vaccines for adolescents, and previously announced that clinical trials found no symptomatic infections among vaccinated children between the aforementioned age range.
According to the findings, the shot appeared to be extremely effective on kids. Pfizer said children produced “strong antibodies” and experienced no serious side effects.
Read also: Covid-19 vaccine for children aged 12 to 15 to be authorised for use ‘by next week’
Although no official statement has been published by health authorities in Qatar, the emergency use approval could ease the return to normalcy for thousands of families in here if the ministry of health goes ahead with a campaign to inoculate adolescents.
In the last weeks of April, Qatar reported an increase of Covid-19 infections in children. On the week of April 18, children in Qatar accounted for 13% of national Covid-19 infections.
The new strains spreading in Qatar are affecting more younger people under the age of 18 than previously seen during the first wave of the virus, the Head of Vaccination at Ministry of Public Health (MoPH) Dr. Soha Al Bayat, told Doha News.
The approval by the FDA could mean the expansion of national vaccination campaigns around the world, providing more immunity to populations.
Follow Doha News on Twitter, Instagram, Facebook and Youtube