The new FDA approved drug Aducanumab, used to treat Alzheimer’s disease, has caused a divide among health experts.
Aducanumab, a new drug for Alzheimer’s disease, was approved by the US’s Food and Drug Administration (FDA) on Monday. Also known by its brand name, Aduhelm, it’s the first treatment to be approved for Alzheimer’s in 20 years. However, experts are divided over whether the drug really works or not.
The disease
Alzheimer’s disease is a progressive cognitive disorder that affects memory and thinking, eventually making even the simplest of tasks, difficult. The condition is the most common cause of dementia in older adults.
It is estimated that approximately 44 million people worldwide are currently living with Alzheimer’s disease, a number expected to rise rapidly in the next few decades. However, only about one in four people with Alzheimer’s get diagnosed as cognitive decline is commonly thought of as a normal sign of ageing.
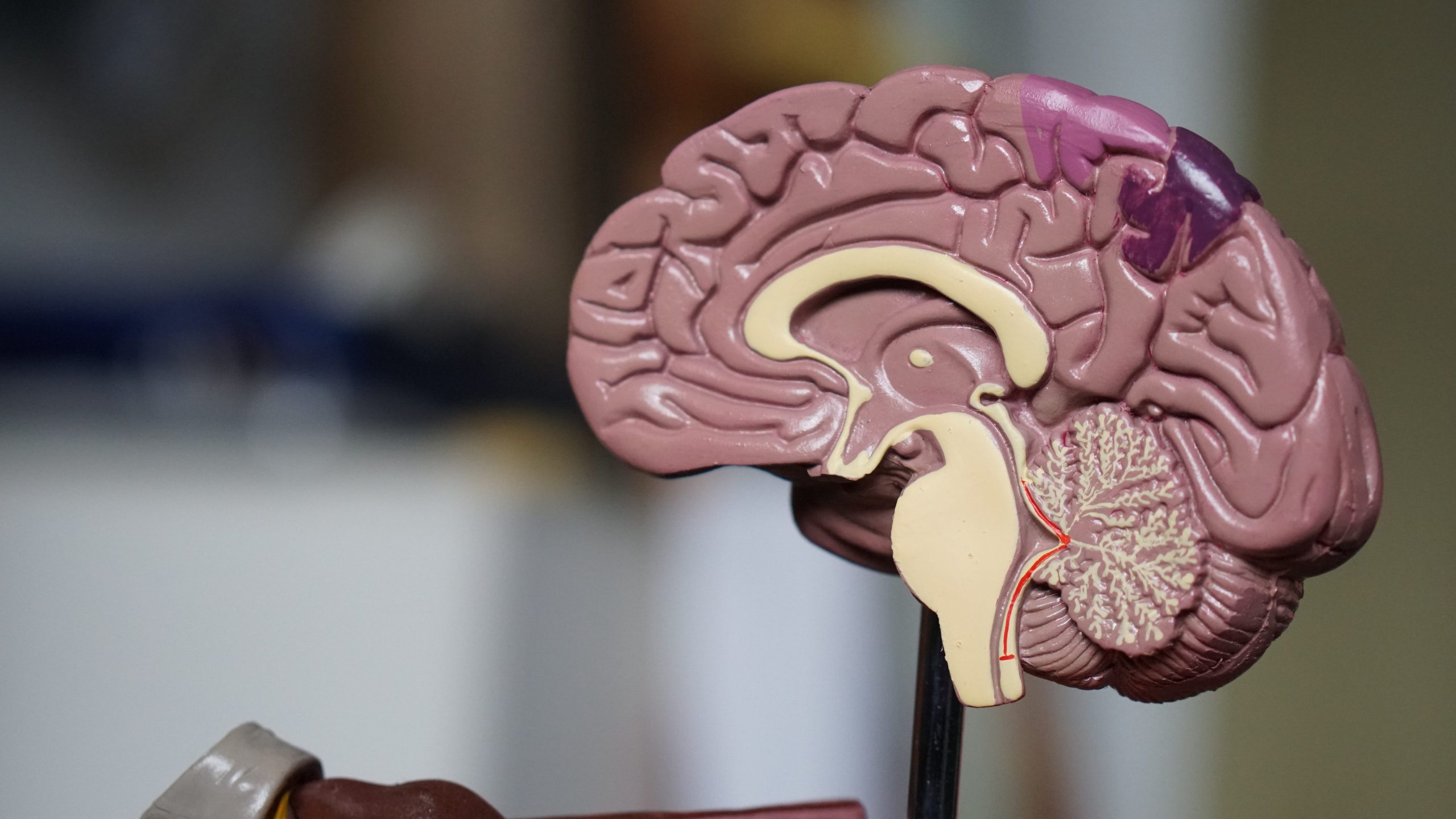
In patients with Alzheimer’s disease, in addition to the noticeable changes in memory and clarity of thought, many changes also occur inside the brain, including the build-up of amyloid plaques and tau-containing neurofibrillary tangles.
These result in the death of brain cells (called neurons) and the destruction of their connections. As neurons get injured and die, and as the connections between their networks disintegrate, many regions in the brain shrink, resulting in a significant loss in brain volume.
Some parts of the brain are more prone to the development of plaques than others, such as the hippocampus. The hippocampus helps us process memories from short-term to long-term, and so damages that occur in this area of the brain are associated with the symptoms of dementia.
Symptoms of Alzheimer’s include:
- Memory loss
- Confusion
- Difficulty or inability to learn new things
- Difficulty with language including reading and writing
- Difficulty in working with numbers
- Difficulty in logical thinking and organizing one’s thoughts
- Decreased attention span
- Difficulty in coping with new situations
- Changes in personality
The drug
Aduhelm, developed by the US-based biotech Biogen, treats Alzheimer’s essentially by attacking amyloid, the protein that forms these abnormal, insoluble clumps in the brains of individuals with the condition. It is this mechanism that makes it the first therapy that targets and affects underlying disease process, rather than the symptoms of Alzheimer’s. It is also the first new treatment for Alzheimer’s to get approved since 2003.
The approval for use has been limited to patients in the very early stages of the disease, with very mild cognitive impairment that has the potential to progress to dementia.
The drug is administered intravenously (by injection) once a month, and it takes approximately one hour for the intravenous infusion. The course of treatment for one year is listed at $56,000 and would have to be administered at a hospital and or clinical setting.
Biogen researchers tested Aduhelm’s efficacy through studies that included approximately 3,500 patients. They found that for patients with mild to moderate Alzheimer’s receiving the treatment for a year, the levels of amyloid were lower than in those who received a placebo.
While the drug is not considered a cure, and does not reverse the progression of the disease, it has been shown to slow the rate of cognitive and functional decline associated with the buildup of amyloid plaques.
The researchers also found that the exact extent of amyloid plaque reduction was dependent on both timing and dosage of the administered drug, while those in the control group, or patients not given Aduhelm, showed no reduction.
The controversy
The approval of Aduhelm has quickly become one of the most debated and controversial decisions of its kind in years. Significant doubts have been raised about the effectiveness of the drug, referring to the evidence provided as insufficient.
This is not the first time the drug’s effectiveness has been questioned. Biogen had already halted two studies of Aduhelm in 2019 due to disappointing results. Several months later, Biogen then announced that the initial analysis of the data was incomplete and that the studies did in fact prove the drug to be effective in slowing down cognitive decline in patients.
Read also: The worrying impact of climate change on human health and wellbeing
Experts have argued that even if the drug had shown a reduction in speed of the cognitive decline in some patients, this benefit could be outweighed by the risk of swelling or bleeding in the brain that had been observed in the clinical trials.
In addition to cerebral edema (swelling of the brain), and bleeding in the brain, Aduhelm was also shown to have the following the side effects: amyloid-related imaging abnormalities, angioedema (swelling under the skin) and urticaria (a reaction resembling hives). Some patients also experience falling, diarrhea and disorientation during clinical trials.
Given the risk of brain swelling and bleeding, patients taking the drug are required to undergo MRIs to monitor their condition and rule out serious side effects, further adding to the time and cost of treatment.
Some have been attributing the accelerated approval due to desperation, given there are so few treatments available for this debilitating, devastating, life-threatening condition. While other drugs currently in clinical trials appear to be more promising, they remain to be at least three years away from potential approval.
However, many have also celebrated the approval, with the Alzheimer’s Association calling the move a historic step forward. Experts have advocated that even small impact is crucial, and could lead the way to a more effective course of treatment down the road.
As such, the FDA will continue to assess Aduhelm and Biogen is required to start a new study to test effectiveness. If clinical benefit is not verified, or if any negative findings surface, the FDA could still retract its approval in the future.
Maha El Akoum, MPH, is a public health professional currently working as Head of Content at World Innovation Summit for Health [WISH].
Follow Doha News on Twitter, Instagram, Facebook and Youtube