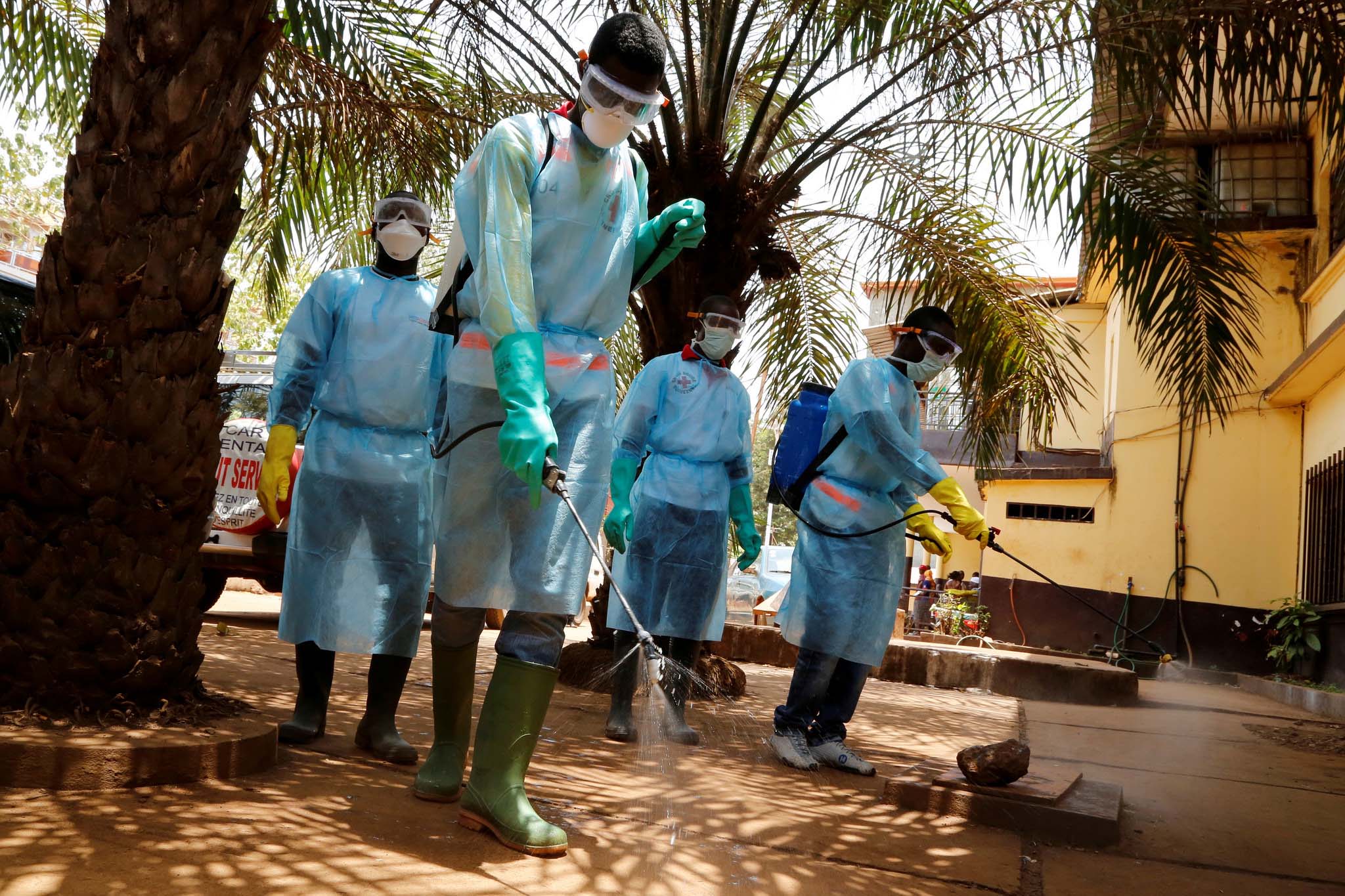
The Ebola epidemic that began in West Africa this year and killed nearly 1,000 people is now officially a global health emergency, the World Health Organization (WHO) has announced.
However, no general bans on international travel or trade were called following a two-day emergency meeting of global health experts in Geneva.
Still, any countries with Ebola transmission, including Guinea, Liberia, Nigeria and Sierra Leone, should declare a national emergency, though should be no general ban on international travel or trade.
In a statement, WHO announced:
“The Ebola outbreak in West Africa constitutes an ‘extraordinary event’ and a public health risk to other States; the possible consequences of further international spread are particularly serious in view of the virulence of the virus, the intensive community and health facility transmission patterns, and the weak health systems in the currently affected and most at-risk countries. (And) a coordinated international response is deemed essential to stop and reverse the international spread of Ebola.
It was the unanimous view of the Committee that the conditions for a Public Health Emergency of International Concern (PHEIC) have been met.”
Ebola re-emergence
According to Qatar’s Supreme Council of Health (SCH), no cases of Ebola have been detected in Qatar since the virus experienced an outbreak earlier this year in West Africa.
As of Aug. 4, WHO said that 932 people have died from the virus and more than 1,711 cases have been reported (1,070 confirmed, 436 probable and 205 suspected).
There is no vaccine for Ebola yet. The virus does not spread through the air like the flu, but only through contact with bodily fluids of someone with the illness.
Symptoms include the sudden onset of fever, muscle pain, headache and sore throat. This can be followed by vomiting and diarrhea, impaired kidney and liver function and internal and external bleeding.
The incubation period for Ebola is two to 21 days, but patients only become contagious once they begin to show symptoms – not during the incubation period, according to the SCH.
On Twitter today, WHO has advised heightened international vigilance of the disease:
Countries shld be prepared to detect, investigate and manage #Ebola cases, incl access to qualified diagnostic lab for Ebola
— WHO (@WHO) August 8, 2014
Committee: Countries w/#Ebola transmission shld conduct exit screening of all persons at intl airports, seaports, major land crossings
— WHO (@WHO) August 8, 2014
Bathing with salt and warm water, drinking water with salt does NOT cure #Ebola. Facts about what helps treat Ebola http://t.co/Evrm7YlsME
— WHO (@WHO) August 8, 2014
Qatar’s response
Health authorities in Qatar have assured residents that necessary precautions are being taken to prevent Ebola from reaching or spreading here.
Speaking previously to Doha News, Peter Cameron, Hamad Medical Corp.’s (HMC) chairman of emergency and medicine, said:
“With Qatar’s airport being a major hub for travelers, we have been on high alert, particularly upon hearing that the virus has traveled by air.
We are always reminding our nursing and medical staff to immediately isolate all ill West Africans who are coming to our hospitals – even if they have small symptoms such as a fever. The initial presentation can be very vague and we are fully aware of this. We are always following WHO and CDC recommendations for dealing with the virus.”
Meanwhile, some regional airlines and airports have put restrictions in place as a way of preventing Ebola from entering its borders.

However, Qatar Airways, which flies to over 140 destinations worldwide, has signaled that business will continue as usual for now. In a statement posted on its website earlier this week, the airline said:
“The health and wellbeing of our passengers and staff is of upmost importance to Qatar Airways and we would like to reassure you that we are monitoring the situation very closely and are in contact with both local and international health and aviation organisations to ensure that all necessary and appropriate measures are in place to protect our passengers, our staff and the general public.”
And speaking to Doha News today, a manager at the airline said:
“We’re constantly monitoring the situation and will update the public as soon as anything changes. WHO has not called for a travel or trade ban as yet.”
Gulf action
Before the international emergency was called, Kuwait warned its citizens against flying to Guinea, Liberia and Sierra Leone. The Kuwaiti Ministry of Health has publicly urged its Ministry of Interior to suspend those from all three countries from entering Kuwait by temporarily stoping the issuance of visas.
Saudi Arabia has also responded to the outbreak by upping its airport screenings and banning Muslim pilgrims from the three countries.
A Saudi man, suspected to be infected by the virus, died at a specialized hospital in Jeddah on Wednesday, according to Saudi health authorities. The man is believed to have caught the virus during a recent business trip to Sierra Leone, although test results have not yet confirmed he had Ebola.
Drugs
Meanwhile, two American aid workers diagnosed with Ebola this week have been flown from Liberia to a hospital in Atlanta to receive treatment.
The two workers are the first to be tested with an experimental drug called ZMapp. So far, doctors have said it’s too early to see results. ZMapp has never been tested on humans, nor has it undergone any clinical trials.
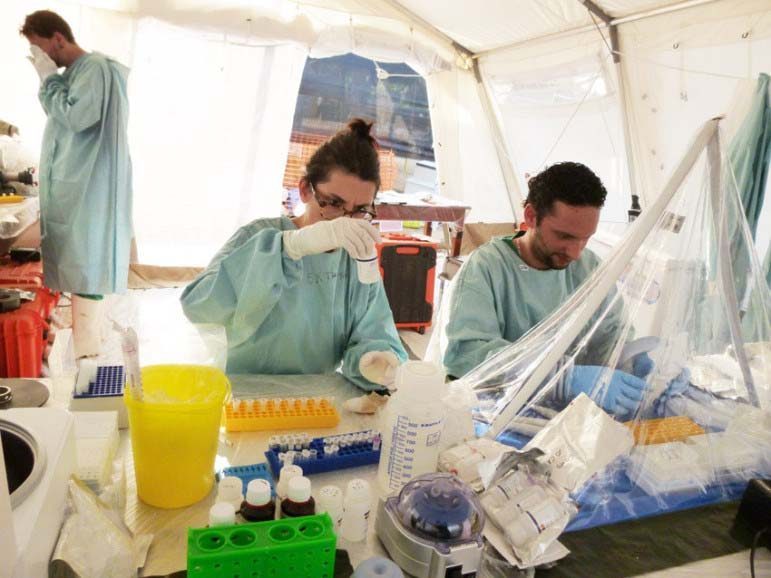
The drug is expected to boost immunity systems so that those who are infected can fight off the virus through the three monoclonal antibodies contained in the serum. The antibodies have been made by lab animals who have been exposed to elements of the virus.
The US government health agency Centers for Disease Control and Prevention (CDC) has made it clear that the drug is not a vaccine that prevents infection, but rather a therapeutic product that can treat those who are afflicted with Ebola.
Many in West Africa have been left asking for doses of the drug in an attempt to put an end to Ebola. But US health organizations like the CDC have stated on their website:
“The product is still in an experimental stage, and the manufacturer reports that there is a very limited supply, so it cannot be purchased and is not available for general use. The manufacturer has been planning for phase 1 clinical trials and does not have the capacity to manufacture large quantities of the treatment. The drug has not gone through clinical trials, meaning its safety and effectiveness has not yet been tested in humans. The manufacturer of the experimental treatment continues to research and evaluate the product’s safety and effectiveness.”
WHO has planned to convene a panel of medical ethicists next week to discuss the ramifications of using an untested drug and who should receive it, given extremely limited supplies.
Read WHO’s full recommendations on how states should handle Ebola here.
Thoughts?